
A-IR is the next-generation respiratory focused clinical research organisation (CRO). We provide a bespoke solution to the assessment and development of new vaccine and therapeutic approaches for respiratory infection, disease and allergy.
Welcome to A-IR
Operating from a state of the art research laboratory in the UK and across a collaborative clinical network of sites across mainland Europe, the US and Asia, we leverage our unparalleled knowledge base, robust study management and broad spectrum biomarker capacity to execute transnational clinical studies and provide clients with the ability to accelerate their own asset development.
We pride ourselves on our considerable experience and detailed understanding of delivering excellence in the academic, government and commercial sectors of respiratory disease and our ability to provide an end-to-end solution.
A-IR CRO Services
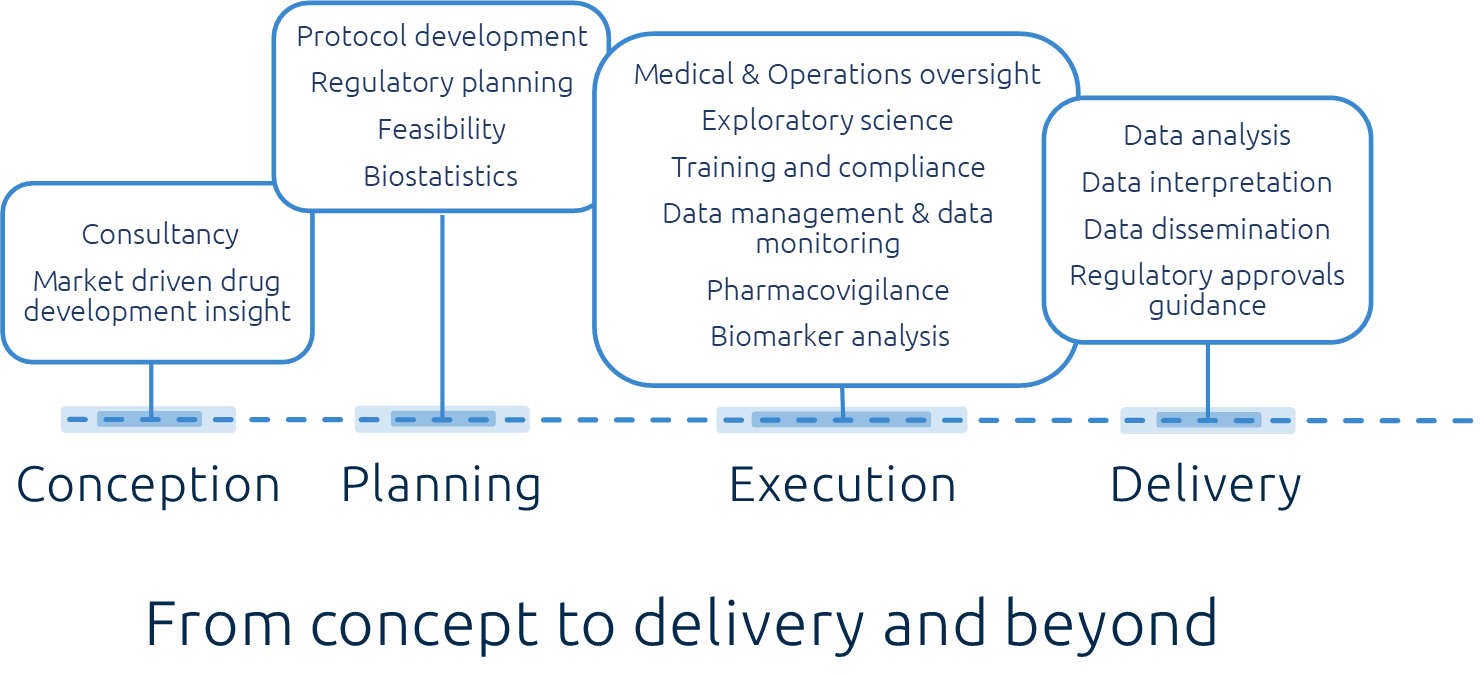
Conception
Creating robust, progressive study designs for investigation of novel prophylactics & therapeutics providing the optimum assessment setting
Approvals
Leading through the undulating regulatory landscape during the study set-up and inception
Support
Support provision: Site feasibility, study monitoring, Biostatisticians & Pharmacovigilance as required
Execution
Execution of the clinical study, exacting precision, integrity & communication throughout
Analysis
State-of-the-art immunology, virology & biomarker analysis, providing Important disease/treatment understanding along with valuable MOA insight intervention
Delivery
Data Insight & interpretation Dissemination through publication and investigatory-led symposia.
Assisting regulatory approvals process
Strategic Commercial Expertise
Pre-clinical and clinical research leadership and management experience with unparalleled access to global scientific expertise
Decades of lifecycle management & leadership expertise
Global pharma and bio business development with capital fund raising experience through our powerful networks
Pharmaceutical pricing, market access and global reimbursement expertise inputs at all stages of research & development programs when required
A-IR Commercial Services
Consult
Pre-clinical and clinical research leadership and management experience with unparalleled access to global scientific expertise
Advise
Decades of lifecycle management & leadership expertise
Capital Raising
Global pharma and bio business development with capital fund raising experience through our powerful networks
Execution
Extensive Global Pharmaceutical pricing and market access inputs at all stages of research & development programs when required
Analysis
State-of-the-art immunology, virology & biomarker analysis, providing Important disease/treatment understanding along with valuable MOA insight intervention
Delivery
Data Insight & interpretation Dissemination through publication and investigatory-led symposia.
Assisting regulatory approvals process
A-IR Solution
A-IR Advantage
Innovative approaches to naturally occurring infection studies, alongside specialism in allergen challenge and live virus challenge models – we offer a unique opportunity to test efficacy of novel treatments and deliver a competitive advantage

Defined virus delivery
Delivery of a defined virus dose, at a defined time point to all subjects, alleviating variables associated with pathogen, seasonality, inflammation kinetics and antiviral immunity
GMP human inoculum
Use of GMP human virus inoculum, including reverse engineered clones, providing the unique ability to determine changes to the pathogen as a consequence of treatment inventions.
Understanding
In-depth understanding of the underlying immunopathology & infection cycle, delivering robust data on a broad range of clinically relevant endpoints
All this culminating to deliver the complete clinical study package
About A-IR
A-IR, your next generation CRO, launched to offer novel opportunities in respiratory indications including live virus challenge with Influenza, Rhinovirus and Respiratory Syncytial Virus, alongside naturally occurring infection studies both in healthy and chronic airways disease populations.
A-IR is led by a world class team of innovators, health science entrepreneurs and seasoned senior pharma and bio corporate leaders. Original fresh thinking, leveraging with reassuring immense depth and breadth of experience and networks in the health care science industry and academia. Our culture sets us apart from other CROs.
Founders Cathryn A. Macrae and Dr Ross P. Walton were also founding members of the first CRO to offer common cold challenge studies in patients with asthma and Chronic Obstructive Pulmonary Disease (COPD) where they pioneered, refined and expanded their expertise in investigative application of virus challenge for the benefit of clients in the commercial setting. They are now joined by David Kwasha in bringing the A-IR advantage to the market.
Cathryn A. Macrae
Co-Founder & Board Director (New York City, USA) a life sciences entrepreneur, has been operating at the inter-section of clinical science and private industry for over 25 years for government, private and public industry, as well as NGOs.
Cathryn has a market-leading ability to form strategic alliances and coalitions with leading sector players, as well as bringing together world-renowned opinion leaders from academia, government and industry. Cathryn has also applied her unique talent and experience throughout her career to identifying and enabling strategic funding pathways across a broad range of health care endeavours.
Ross P. Walton MSc. PhD.
Co-Founder & Board Director (London, UK) has a PhD from Imperial College specialising in respiratory viral infections and their interplay with inflammatory airways disease. A cellular immunologist by training, Ross has over 17 years of experience in developing viral challenge models and employing state-of-the-art analytical approaches in patient samples. In addition to his scientific expertise, academic network and knowledge base, Ross has unique strategic capabilities in business development, lifecycle management and leadership.
David Kwasha
Managing Director & Head of Asia Pacific Region (Sydney, Australia) has forged a unique and globally successful 30 year career in senior leadership in the pharmaceutical industry. His experience has traversed medical affairs, R&D, marketing, market access, business development, commercial operations and senior executive leadership at local, regional and global levels. Following a stellar 10 year career with Roche where David achieved renown performance as a Global Business and Lifecycle Management Leader, he spent the last 20 years as a pioneering leader for new rare disease companies such as Actelion and Alexion from start- up to global operational success.
Insight and case specific research and clinical science expertise is enhanced by a committed network of true, key world opinion leaders in respiratory infection and disease.

